Acid Base Equilibrium Practice Test
The anion gap is the difference between the number of cations versus anions. Polylactic acid is a biobased and compostable thermoplastic polyester that has rapidly evolved into a competitive commodity material over the last decade.

Acid Base Questions Practice Khan Academy
In chemistry pH p iː ˈ eɪ tʃ historically denoting potential of hydrogen or power of hydrogen is a scale used to specify the acidity or basicity of an aqueous solutionAcidic solutions solutions with higher concentrations of H ions are measured to have lower pH values than basic or alkaline solutions.
. The problems have been color-coded to indicate whether they are. Chemical processes Acidbase equilibria. HCl H 2 O H 3 O Cl This answer choice contains one of the strong acids from our list so we can immediately eliminate this answer choice.
HPO 4 2 H 2 O H 3 O PO 4 3 This answer choice shows a negatively charged ion losing an additional proton. Equilibrium Constant of an Electrochemical Cell Reaction. One can understand quickly that the narrower the peak low W the higher N and thus the efficiency.
Test your MCAT readiness with these MCAT Chemistry practice questions. When youre performing pH calculations always make sure your answers make sense. H 2 C 2 O 4 CH 3 COOH lactic acid phosphoric acid acetic acid or vinegar etc.
Practice what youve learned and study for the AP Chemistry exam with more than 70 AP-aligned questions. Organic Chemistry Practice Problems at Michigan State University. Where t R refers to the retention time of the peak and W b refers to the peak width at baseline in Equation 8-2 and W h its width at half-height in Equation 8-3.
Learn the Temperature Where Fahrenheit and Celsius Are the Same. Are a few examples of weak acids. This unit examines the role of chemical equilibrium in acidbase chemistry.
This is unlikely where we want a large negative charge on an ion. The strength of weak acid is measured by the amount of dislocation in it-the more it dissociates the stronger the acid becomes. Sulphuric acid is a strong acid and solutions will typically have pHs of around 0.
Weak acids are acids that do not dissociate entirely in solution. An anion gap can be high normal or. Because it is only a weak acid the position of equilibrium will lie to the left.
It is designed to help students link chemical computations with authentic laboratory chemistry. The -COO-group is a weak base and takes a hydrogen ion from a water molecule. A weak acid in other terms is an acid that is not strong.
For example in its aqueous solution NaCl undergoes complete ionization yielding sodium ions. Your body maintains balance by holding onto or releasing carbon dioxide through the lungs acid or bicarbonate through the kidneys base. Vital electrolytes are compounds that ionize entirely upon dissociation in their ionic solution whereas weak electrolytes only dissociate partially.
Please scroll below to find our collection of. A carboxylic acid in solution will exist in equilibrium with carboxylate or acetate its conjugate base. The -NH 3 group is a weak acid and donates a hydrogen ion to a water molecule.
The salt solution acid solution base solution and so on. The following problems are meant to be useful study tools for students involved in most undergraduate organic chemistry courses. H 2 SO 4 aq H 2 Ol H 3 O aq HSO 4-aq The second hydrogen is more difficult to.
Practice Chemistry with Worked Chemistry Problems. The amine dissolves in the reagent phase and. Disregard the complex ICE charts for calculating.
The Virtual Lab is an online simulation of a chemistry lab. Figure 8-3 illustrates the values used with these equations. The anion gap AG is a measure of acid-base balance.
The Hinsberg test is conducted in aqueous base NaOH or KOH and the benzenesulfonyl chloride reagent is present as an insoluble oil. Convert Temperatures From Kelvin. Because of the heterogeneous nature of this system the rate at which the sulfonyl chloride reagent is hydrolyzed to its sulfonate salt in the absence of amines is relatively slow.
The Cowpers glands produce. Solid and weak electrolytes are two types of electrolytes in ionic equilibrium. While its theoretically possible to calculate a negative pH pH values should be between 0 and 14 in practice.
Strong acids dissociate completely. Although this reaction also fits the criteria for a double- displacement reaction choice C in which two molecules essentially exchange ions with each other neutralization is a more specific description of the process. Sperm are produced in the seminiferous tubules of each testis.
Chemistry of buffers and buffers in our blood. More information and offline downloads. Most likely to be useful to students in year long rather than survey courses 3.
Test prep MCAT Foundation 5. The acid reacts with water to give a hydroxonium ion a hydrogen ion in solution if you like and a hydrogensulphate ion. The lab allows students to select from hundreds of standard reagents aqueous and manipulate them in a manner resembling a real lab.
Ka and acid strength. Chapter 27 - Ions And Ionic Compounds Chapter 28 - Naming Inorganic Compounds Chapter 29 - Some Simple Organic Compounds Chapter 3 - Chemical Reactions And Reaction Stoichiometry Chapter 31 - Chemical Equations Chapter 32 - Simple Patterns Of Chemical Reactivity Chapter 33 - Formula Weights Chapter 34 - Avogadros Number And The Mole Chapter 35 - Empirical. Learn about pH and pOH weak acids and bases buffers acidbase titrations and more.
Quiz Yourself Using These 20 Practice Chemistry Tests. What Filtration Is and How Its Done. Again the equilibrium lies to the left.
The scrotal sac is located at the base of the penis and contains the testes the epididymis the external spermatic fascia and the spermatic cord. This means that a. One key bottleneck in extending the use of PLA is the control of its crystallinity.
Unit 2 Equilibrium Unit 3 -Solubility Unit 4 - Acids Bases and Salts Unit 5 - Redox Review Exams TRY OUT ONLINE TUTORING FOR FREE. An acid should have a pH much less than seven usually one to three and a base should have a high pH value usually around 11 to 13. When you dissolve an amino acid in water both of.
In which an acid and a base react to form a salt and usually water. The seminal vesicles secrete many components of semen. The higher the plate number N the greater the efficiency of the column.
Cations are positive base and anions are negative acid. Sperm are then stored in the epididymis to mature. The pH scale is logarithmic and inversely indicates the.
If you find your acidbase knowledge weak start by reviewing my acidbase video series reading this link acidbase overview article and downloading my free acidbase cheat sheet. ALSO SEE MY CHEM 11 and SCIENCE 10 SITES. What Really Happens If You Put Sugar in a Gas Tank.
Most likely to be. This is the currently selected item. Understanding the crystallization behavior is particularly crucial to control PLAs degradation rate thermal resistance as well as.
This reaction is virtually 100 complete.

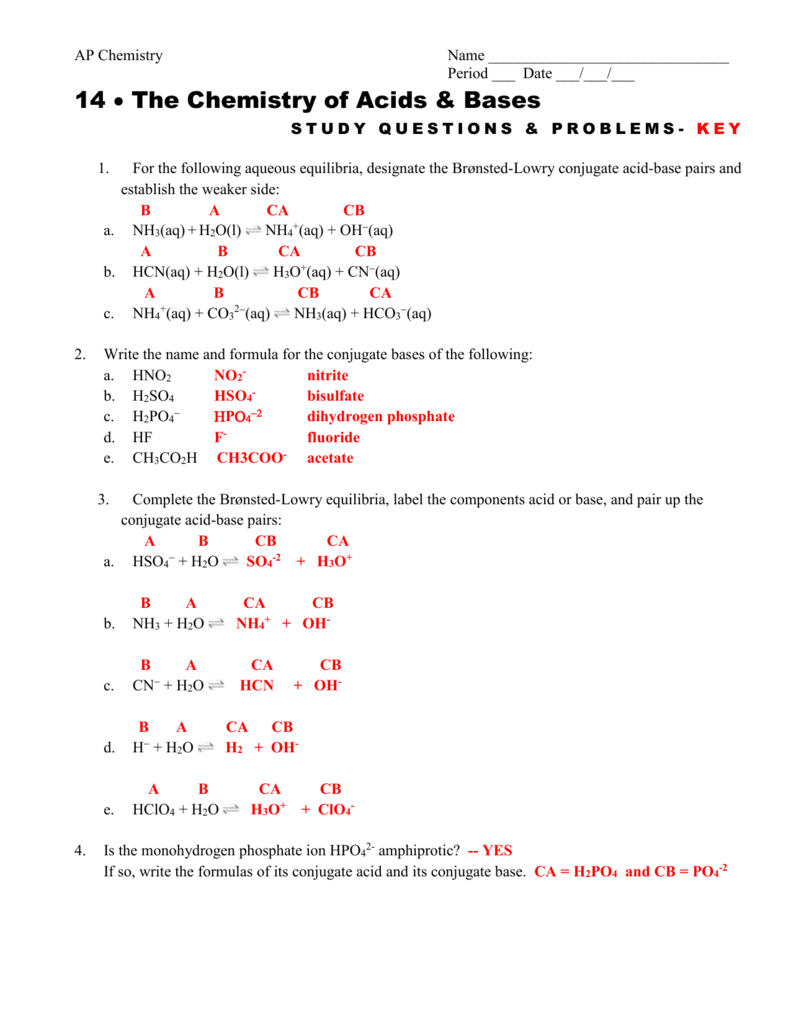
Chapter 17 Study Questions And Problems
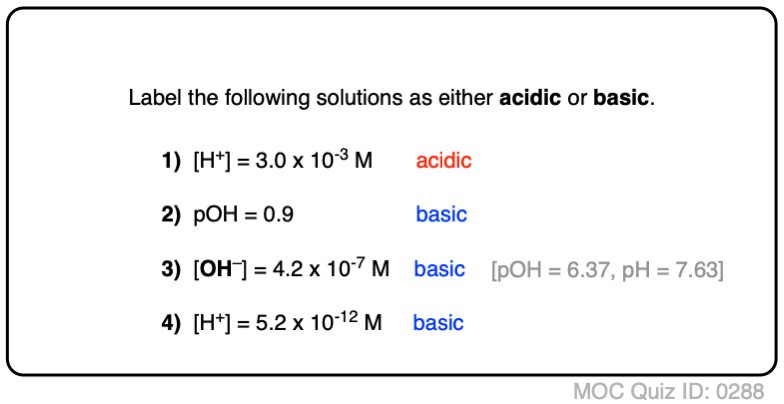
Acid Base Practice Problems Master Organic Chemistry
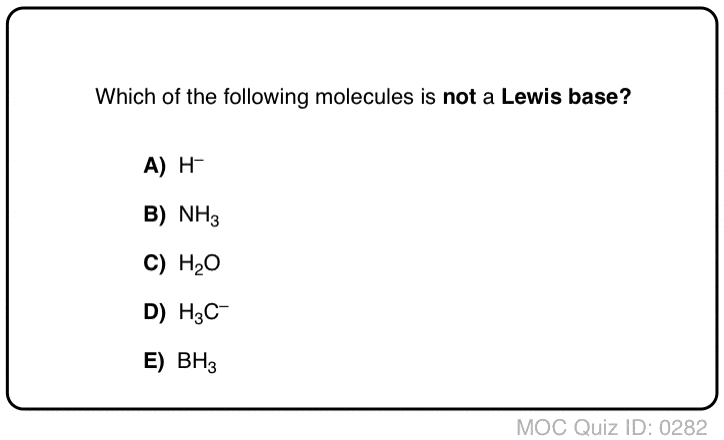
Acid Base Practice Problems Master Organic Chemistry

Acid Base Equilibrium Practice Organic Chemistry Youtube
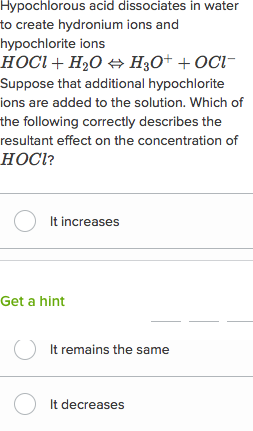
Acid Base Questions Practice Khan Academy

Acid Base Equilibrium Organic Chemistry Practice Questions
0 Response to "Acid Base Equilibrium Practice Test"
Post a Comment